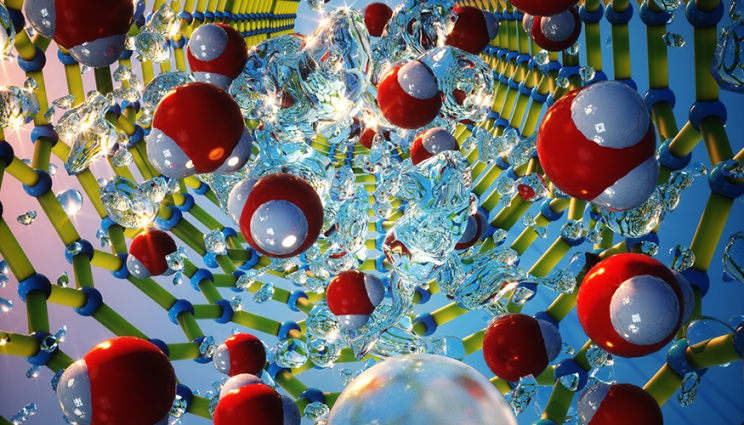
Machine learning reveals refreshing understanding of confined water
LLNL scientists combined large-scale molecular dynamics simulations with machine learning interatomic potentials derived from first-principles calculations to examine the hydrogen bonding of water confined in carbon nanotubes (CNTs). They found that the narrower the diameter of the CNT, the more the water structure is affected in a highly complex and nonlinear fashion. The research appears on the cover of The Journal of Physical Chemistry Letters. The hydrogen-bond network of confined water in nanopores deviates from the bulk liquid, yet looking into the changes is a significant challenge. In the recent study, the team computed and compared the infrared spectrum of confined water with existing experiments to reveal confinement effects. “Our work offers a general platform for simulating water in CNTs with quantum accuracy on time and length scales beyond the reach of conventional first-principles approaches,” said LLNL scientist Marcos Calegari Andrade, lead author of the paper. Read more at LLNL News.